Abstract
Prostate cancer (PC) is the most common cancer in man (Int. J. Urol. 2018, 25, 220). It is therefore desirable to understand the biological mechanisms, identify new targets for a development of new strategies to combat PC, and last but not least to develop medicaments, which would show a selective cytotoxicity via interaction with these targets, preferably with no interference with healthy cells. During this project, new molecules will be prepared and evaluated for their selective cytotoxicity towards castration resistant metastatic PC carcinoma (PC-3). It is hypothesized that a novel mode of action is responsible for the effect of the molecules. The hypothesis and the design of the molecules results from our preliminary wet laboratory and in silico studies.
Preliminary results
Natural products selagibenzophenones A, B, and C (Catalysts 2021, 11, 708. Eur. J. Org. Chem. 2022, e202200014) were prepared in our laboratory as well as 11 unnatural derivatives. Compounds were evaluated for their cytotoxic properties at prostate (PC-3), colon (HT-29), breast cancer (MCF-7), and healthy cells (HUVEC). A lead candidate SMR036 was identified with cytotoxicity towards PC-3 cell lines with the activity in low micromolar range a low toxicity towards healthy cells, and with selectivity index up to 19 (cytotoxicity towards healthy/PC cells). In silico studies were carried out resulting into identification of two possible molecular targets, namely PDE42D and PARP1. A series of now dual inhibitors of the above enzymes was designed, using molecular docking. The proposed derivatives are expected to bind to both receptors and should have a good druggability resulting from ADMET analysis (Figure 1, unpublished results).
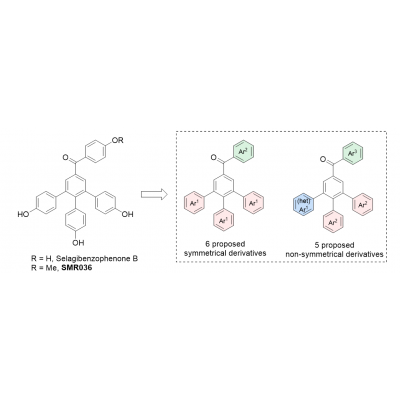
Figure 1
Aims of the project
The proposed symmetrical as well as non-symetrical derivatives will be prepared in the laboratory of the applicant by the postdoc and evaluated for the cytotoxic properties in the laboratory of collaborator (prof. Tugba Tumer). The inhibitory properties towards the identified targets will be evaluated. The role of the dual inhibitory effect on the selective cytotoxicity will be examined.
Design of the research
In the first stage, the designed molecules will be synthesized. The experience, previously gained, will be utilized within the preparation of the molecules with the symmetrical substitution pattern. The synthesis will commence from methyl gallate, which will be converted to triflates and cross-coupling reaction, followed by reduction of the ester moiety to aldehyde, addition of organomethylated species and oxidation to ketone (Scheme 1, upper sequence). For the synthesis of the non-symmetrical molecules, masking of the ortho-phenolic groups as acetals (e.g. in the reaction with acetone), further cross-coupling for introduction of moiety Ar1, deprotection, and decoration with moieties Ar2 will assure the access to the desired non-symmetrical derivatives (Scheme 1, bottom sequence).
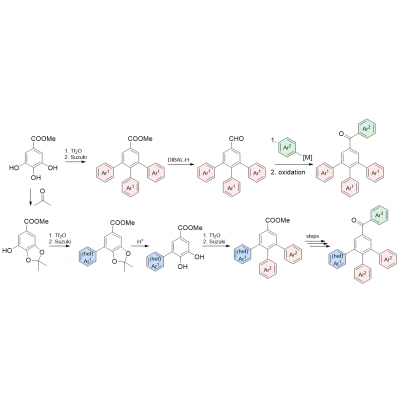
Scheme 1
In the later stage, the derivatives will be evaluated for their cytotoxic properties, as well as for their inhibitory properties towards the both targets of interest, in order to evaluate the above stated hypothesis. The evaluation will be carried out in a collaboration with prof. Tugba B. Tumer, who is (Çanakkale Onsekiz Mart University) with whom the collaboration was established and the preliminary data were collected.
Significance
The fulfilling the aims of the project will have an impact on the treatment of the castration resistant metastatic PC. First, successful demonstration that the dual inhibition of the PDE42D and PARP1 leads to a selective toxicity towards PC cells will represent a new therapeutic strategy and a novel treatment approach for the PC. The synthesized compounds will be new potential anti-cancer drugs with such a mode of action.
I declare that:
● co-founding 1000 EUR/month is ensured
● project is approved by head of corresponding department
Contact details:
Dr. Lukas Rycek, MSc.
Department of Organic Chemistry, Faculty of Science, Charles University
email: rycekl@natur.cuni.cz
telephone number: (+420) 22195 1981