Project proposal and aims:
Transient post-translational modification that is largely neglected in proteomic approaches is the formation of imines (Schiff bases) of lysine residues with carbonyl (aldehyde and ketone) containing metabolites (Fig.1). A prototypical example of this interaction is pyridoxal phosphate and retinal cofactor binding in transaminases and opsins.[1,2] However, recent research indicates that this type of interaction might be more prevalent and important than previously thought. For instance, the intimate interaction of MHC-like proteins with carbonyl-containing metabolites can have a major effect on the immune response to microbial infections and malignant tumors.[2,3] The full scale of the possible metabolites presented to T-cells through this interaction is largely unknown.
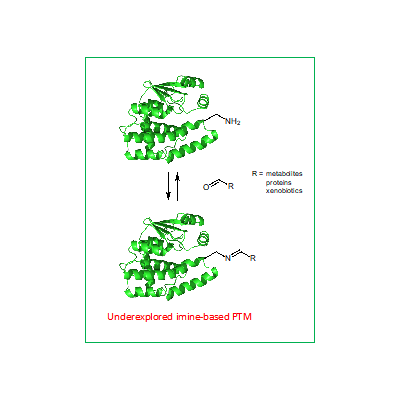
The aim of the proposal is the development of a chemical probe for selective labeling of imines in proteomes and the identification of specific proteins and metabolites involved. The design relies on the chemical reaction of imines with organic phosphites that generates a covalent adduct that can be further processed. Such a tool would enable fundamental insight into the metabolic surveillance of immune system that has also a significant translational potential.
References:
[1] A. C. Eliot, J. Kirsch, Annu. Rev. Biochem. 2004, DOI 10.1146/ANNUREV.BIOCHEM.73.011303.074021.
[2] L. O. Björn, Photobiology: The Science of Light and Life, Springer, 2015.
[3] L. Kjer-Nielsen, O. Patel, A. J. Corbett, et al., Nature 2012, 491, 717.
[4] A. J. Corbett, S. B. G. Eckle, R. W. Birkinshaw, et al., Nature 2014, 509, 361.
I declare that:
● co-founding 1000 EUR/month is ensured
● project is approved by head of corresponding department
Co-founding resources: 2700-110-112700
Contact details:
Jiří Míšek, PhD.
department: Department of Organic Chemistry, Faculty of Science, Charles University.
email: misek@natur.cuni.cz
telephone number: +420 777 148 178