Study of electrochemical and adsorption processes on nanostructured sp3 carbon surfaces for rational designing of electrode surfaces for electroanalysis
Research group: UNESCO Laboratory of Environmental Electrochemistry
Leader of the research group: Prof. RNDr. Jiří Barek, CSc. (barek@natur.cuni.cz)
Project leader: RNDr. Karolina Schwarzová, Ph.D. (kpeckova@natur.cuni.cz)
https://www.facebook.com/UNESCO.Electrochemistry/?ref=page_internal
Project summary:
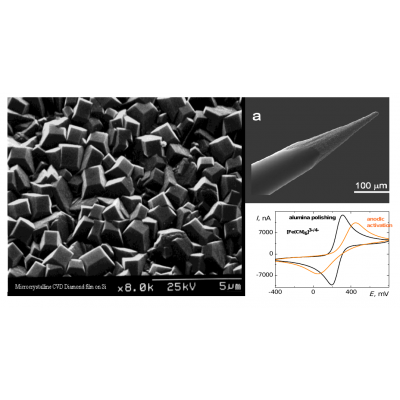
At present, different carbon-based materials and nanomaterials are produced by either top-down or bottom-up approaches. Many reports are devoted to applications of these materials in electrochemical analysis of a wide range of organic molecules that are used in medicine or industry. The majority of these studies include more or less empirical modifications production and pretreatment processes to improve detection limits and/or selectivity for specific groups of analytes. Among carbon based materials, boron-doped diamond (BDD) attracts attention due to its advantageous properties including high chemical and mechanical stability, biocompatibility, wide potential window, especially in anodic region, and low background currents. Similarly as for the sp2 carbon, properties of BDD are influenced by surface termination given by electrochemical (frequently used anodization to incorporate oxygenous surface groups, or cathodization to achieve surface termination with H atoms) and/or mechanical (manual polishing treatment of deposited BDD films. The type of surface pretreatment influences strongly the hydrophobicity/hydrophilicity of both sp2 and sp3 surface and influences the polarity of the surface bonds.
Within this context, the proposed project aims to study of the interaction of nanostructured carbon-based materials with focus on sp3 carbon surfaces with simple organic molecules, heterocyclic compounds, and biomolecule, focusing on details in the interplay among surface termination, morphology, electron transfer kinetics, and adsorption of the selected compounds. The main goal of the project is a complex understanding and description of electrochemical and adsorption processes at the electrolyte-electrode interface and extension of the knowledge for rational design of electrode surfaces.
To achieve the goal, we propose to solve the following specific aims.
1) Characterization of physico-chemical properties of natural and artificially prepared nanostructured sp3 carbon surfaces.
2) Study of the interaction between nanostructured sp3 carbon surfaces and model organic compounds at the electrolyte-electrode interface
3) Tuning of sp3 carbon surface properties to achieve efficient adsorption and fast electron transfer of selected groups of compounds, providing base for development of sensitive, selective, and low-cost methods based on voltammetric ar amperometric techniques and construction of electrochemical biosensors.
The proposed project is based on close cooperation of UNESCO Laboratory of Environmental Electrochemistry, Faculty of Science, Charles University, Prague), Institute of Physics a of the Czech Academy of Sciences ASCR, Institute of Biophysics of ASCR, and the laboratory of Prof. G. M. Swain (Michigan State University, East Lansing, U.S.A.).
Relevant publications of the project leader
S. Baluchová, A. Daňhel, H. Dejmková, V. Ostatná, M. Fojta, K. Schwarzová-Pecková, Recent progress in the applications of boron doped diamond electrodes in electroanalysis of organic compounds and biomolecules – A review, Anal. Chim. Acta, 1077 (2019) 30-66.
S. Hason, A. Danhel, K. Schwarzova-Peckova, M. Fojta, Carbon electrodes in electrochemical analysis of biomolecules and bioactive substances: roles of surface structures and chemical groups, in: D.P. Nikolelis, G.-P. Nikoleli (Eds.) Nanotechnology and Biosensors, Elsevier, Amsterdam, Oxford, Cambridge, 2018, pp. 51-111.
Financial support: Czech Science Foundation, project GACR 20-03187S
Deadline is closed