Project summary:
Cytokine immunocomplexes (non-covalent complexes of given cytokine and a corresponding monoclonal antibody recognizing that cytokine) and immunocytokines (fusion proteins composed of a cytokine and a monoclonal antibody, in some cases recognizing that same cytokine, but not always, generally) are being intensively studied and developed for their enhanced properties. Cytokines are usually short-lived molecules with only a brief half-life in blood circulation. The antibody confers them much longer half-life, but not only this – it can also modulate their binding to cytokine receptors on various populations of immune cells, thus potentially leading to selective stimulation and expansion of certain desired cell types, such as effector cells in the context of tumor immunotherapy, or suppression of unwanted cell types, like immunosuppressive regulatory cells. In other instances, the monoclonal antibody is the main part of the fusion molecule, and its properties are further enhanced by the addition of a stimulatory cytokine, e.g., to overcome an immunosuppressive environment within a solid tumor.
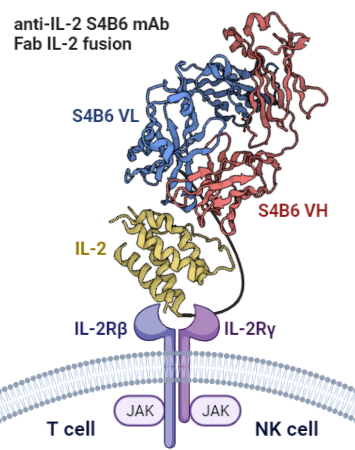
Among various cytokines, interleukin-2 (IL-2) holds a prominent position as one of the best known and much studied immunostimulatory cytokines, which is critical for T cell proliferation, effector responses, and establishment of T cell memory. It is vital for regulating immune homeostasis in terms of generation, expansion, and survival of regulatory T cells. IL-2 also plays a role in the proliferation and cytolytic activity of natural killer (NK) cells. This project is based on the ongoing successful collaboration with Dr. Jakub Tomala (IBT CAS), Dr. Marek Kovář (IMB CAS), and prof. Jamie Spangler (Johns Hopkins University, USA) to develop and characterize IL-2 immunocytokines (ICs) usable for immunotherapy by providing selective expansion of effector T cells and NK cells. Within this PhD project, further target ICs, such as IL-15 IC selectively stimulating NK cells, and their combinatorial use in selected mouse tumor models will be studied.
Structural description of protein-protein interactions is the cornerstone of knowledge enabling detailed molecular understanding of processes such as receptor-ligand recognition within the immune system, potentially leading to a design of targeted immunotherapeutics or drugs in general. Precise analysis of such interacting systems usually relies on X-ray structural analysis, which is dependent on the successful preparation of diffracting crystals of the given protein complex – a task significantly more difficult for flexible or large protein complexes/fusion proteins. On the contrary, biophysical methods such as AUC, ITC, SPR, MST, or small-angle X-ray scattering enable the study of protein interactions directly in solution, with cryoEM as a further option to assess the structure of large protein assemblies. These methods will be employed to characterize ICs and their interactions with the respective IL receptor subunits and thus contribute to a better understanding and design of these promising immunotherapeutics.
Profile of an ideal candidate:
MSc. or equivalent in Biochemistry, Molecular Biology, Immunology, or a related field, and a good knowledge of English is required. Experience with DNA cloning and recombinant protein expression and purification, protein-related biophysical and structural biology methods, or flow cytometry and cell-based immunological assays is an advantage.
Literature: