Project summary:
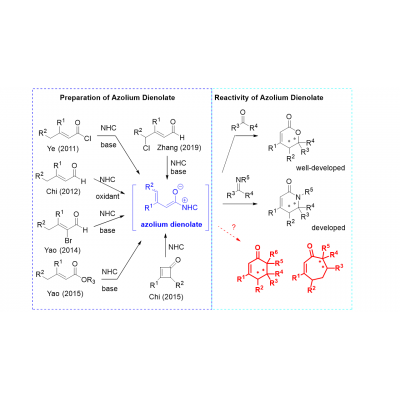
Asymmetric organocatalysis has grown into one of the pillars of asymmetric catalysis, employing various small organic molecules as efficient catalysts, including N-heterocyclic carbenes (NHC). NHCs can efficiently catalyze transesterifications, acylations, polymerizations, and other reactions, and they also found widespread application as ligands in transition metal catalysis. Whereas NHCs containing imidazolylidene framework dominate as ligands in transition metal catalysis (to stabilize coordinatively unsaturated active species), triazolylidene carbenes are the main players in catalytic processes using NHC as catalysts. The vast majority of enantioselective transformations catalyzed by NHCs proceed through the umpolung of aldehydes. In the last few years, azolium dienolate as an interesting intermediate generated from carbonyl compounds and NHC, has received attention as an alternate strategy for remote functionalization (γ-position of enals). We have recently developed new type of enantioselective formal cycloadition reaction based on azolium dienolate chemistry. The goal of the project is to develop new enantioselective carbon-carbon bond-forming reactions and the construction of biologically relevant (hetero)cyclic molecules based on azolium dienolates. Requirements: M.Sc. degree in organic chemistry, organometallic chemistry or related fields, experience with synthesis and characterization of small molecules, strong interest in organic synthesis and catalysis, open to learning new techniques and working in a team, good knowledge of English.
Selected recent publications of the research group:
1. Lóška, L.; Dočekal, V.; Císařová, I.; Veselý, J. Org. Lett. 2023, 25, 174–178. " Stereoselective N-Heterocyclic Carbene Catalyzed Formal [4+2] Cycloaddition: Access to Chiral Heterocyclic Cyclohexenones." doi.org/10.1021/acs.orglett.2c04021
2. Kamlar, M..; Urban, M.; Veselý, J. Chem. Rec. 2023, e202200284. "Enantioselective Synthesis of Spiro Heterocyclic Compounds Using a Combination of Organocatalysis and Transition-Metal Catalysis.” doi.org/10.1002/tcr.202200284
3. Bhosale, V. A.; Císařová, I.; Kamlar, M.; Veselý, J. Chem. Commun., 2022, 58, 9942–9945. "Catalytic asymmetric addition to cyclic N-acyl-iminium: access to sulfone-bearing contiguous quaternary stereocenters." doi.org/10.1039/D2CC02667H